Combustion Analysis
Last Updated 10/2025
English
30-Day Money Back Guarantee
Full Lifetime Access
Finish in
60 mins! Run Time
60 mins! Run Time
Made for for
Employees
and
Supervisors
Employees
and
Supervisors
No Certificate
Provided
Provided
Mobile -
Friendly
Access
Friendly
Access
What you'll learn
In this course, the student gets familiar with the ideal combustion and its h - T diagram
Ideal operation and major performance trends
Description
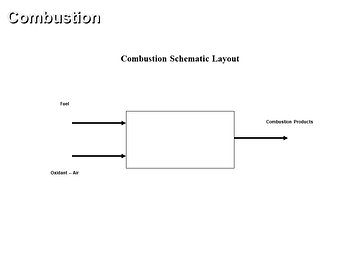
Combustion is a process of active oxidation of combustible compounds such as: carbon, hydrogen and sulfur. Therefore, combustion is a chemical reaction. High amount of heat is released during the combustion process. Combustion has a high degree of importance in engineering.
Complete and adiabatic combustion of carbon, hydrogen, sulfur, coal, oil and gas at constant pressure, with no heat loss, with standard air as the oxidant at stoichoimetric conditions is presented. In this basic combustion course, both fuel and oxidant have the input temperature of 298 [K] or 77 [F]. Therefore, there are six separate combustion cases covered in this basic combustion online course. Reactants and combustion products specific enthalpy values change with an increase in the temperature and such specific enthalpy values are presented in a plot where one can notice the flame temperature definition. Physical properties of basic combustion reactants and products species are presented in a specific enthalpy vs temperature plot. For each combustion case considered, combustion products composition on both weight and mole basis is given in tabular form and plotted in a few figures. Also, flame temperature, stoichiometric oxidant to fuel ratio and fuel higher heating value (HHV) are presented in tabular form and plotted in a few figures. The provided output data and plots allow one to determine the major combustion performance laws and trends.
Table of Contents
Combustion
Analysis
Assumptions
Governing Equations
Input Data
Results
Conclusions
Frequently Asked Questions
This course is designed for employees and supervisors who need to complete Combustion Analysis training
Yes. This course is designed to meet applicable federal requirements and commonly mandated state standards. Always confirm specific state or industry requirements with your local regulations.
The course takes approximately 60 minutes to complete and can be paused and resumed at any time.
No. This course does not include a certificate of completion.
Yes. You can assign this course to individuals or groups using Coggno’s LMS, or purchase multiple seats for your team.
Yes. This course can be exported for delivery in most learning management systems (SCORM compatible).
Yes. The course is fully self-paced and available 24/7.
Yes. This course includes a knowledge check to reinforce learning and verify completion.
Learners have lifetime access from the date of purchase.
Yes. A preview is available so you can review the course format and content before purchasing.
Yes. Content is reviewed and updated as regulations and best practices change.
No. This course is not included with the Prime Subscription and must be purchased separately.
Yes. Refund requests can be submitted within 30 days of purchase.